Amarna has developed a broad product pipeline based on the fundamental features of its proprietary Nimvec™ gene delivery vector platform: Safe, non-immunogenic and tolerance inducing delivery and long term expression of transgenes in humans.
Induction of tolerance towards specific self-proteins that are primary self-antigens in autoimmune diseases. Nimvec™-mediated delivery of primary self-antigens to a tolerizing setting, induces tolerance to the self-antigens to reestablish immune homeostasis and resolve the autoimmune condition.
Down-regulation of inflammation in chronic inflammatory diseases, by localized delivery of anti-inflammatory and regeneration promoting transgenes. Our current project is targeting a generic mechanism which will be applicable for a range of inflammatory indications.
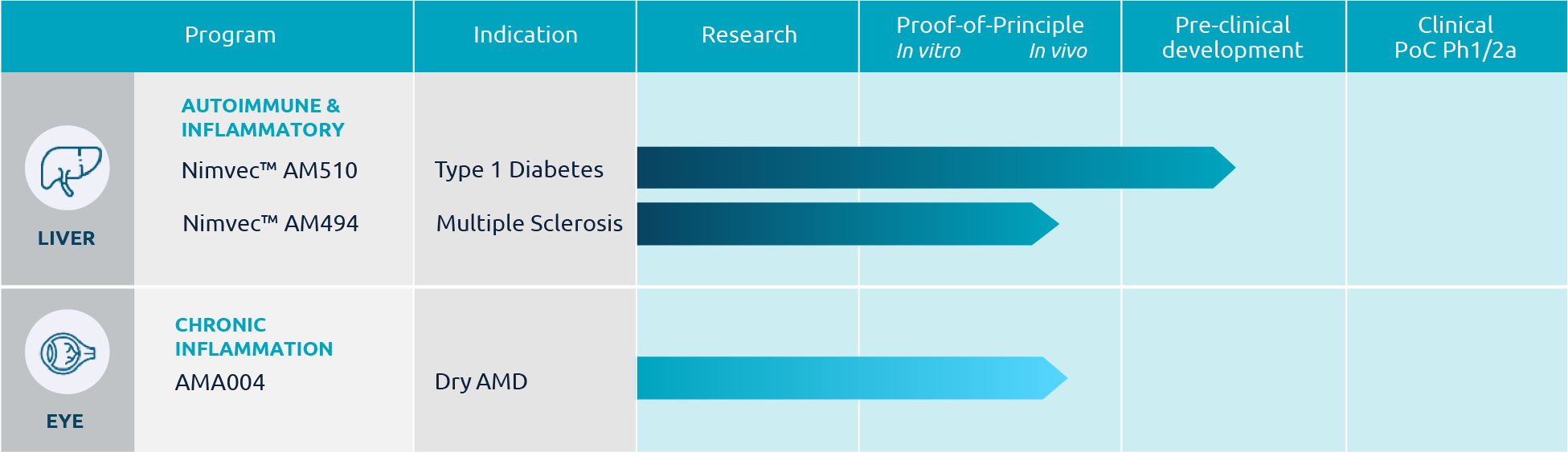
Type 1 Diabetes
Nimvec™ AM510 is a tolerance inducing gene therapy for the treatment of Type 1 Diabetes. Diabetes is an autoimmune disease where self-reactive T lymphocytes selectively attack and destroy insulin-producing β cells lodged within the pancreas, leaving the patient unable to maintain glucose homeostasis. Proinsulin (PI) is considered to be the primary self-antigen involved in the autoimmune β cell destruction. To date, Type 1 Diabetes cannot be cured and the glucose homeostasis can be more or less maintained in patients by daily insulin injections. Although Diabetes is seen as a manageable disease nowadays, secondary complications of the current therapy are considerable and lead to significant morbidity and mortality. Using Nimvec™ AM510 we intend to restore the immune tolerance to proinsulin and potentially cure the patients.

Multiple Sclerosis
AMA003 is a aim to develop a novel treatment of Multiple Sclerosis (MS). MS is an autoimmune disease where self-reactive T lymphocytes selectively attack and destroy oligodendrocytes leading to demyelination of axons in the brain and spinal cord that finally results in functional disability and premature death. Myelin components such as myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP) are considered to represent the primary self-antigens involved in the chronic autoimmune destruction of oligodendrocytes. To date, MS cannot be cured. The current treatment options include the use of immune-suppressing antibodies to prevent the recurrence of acute attacks and to delay disease progression. However, long-term use of general immuno-suppressing drugs coincides with severe adverse side effects. Using AMA003 we intent to restore the immune tolerance to MOG and MBP and potentially cure the patients from the disease.